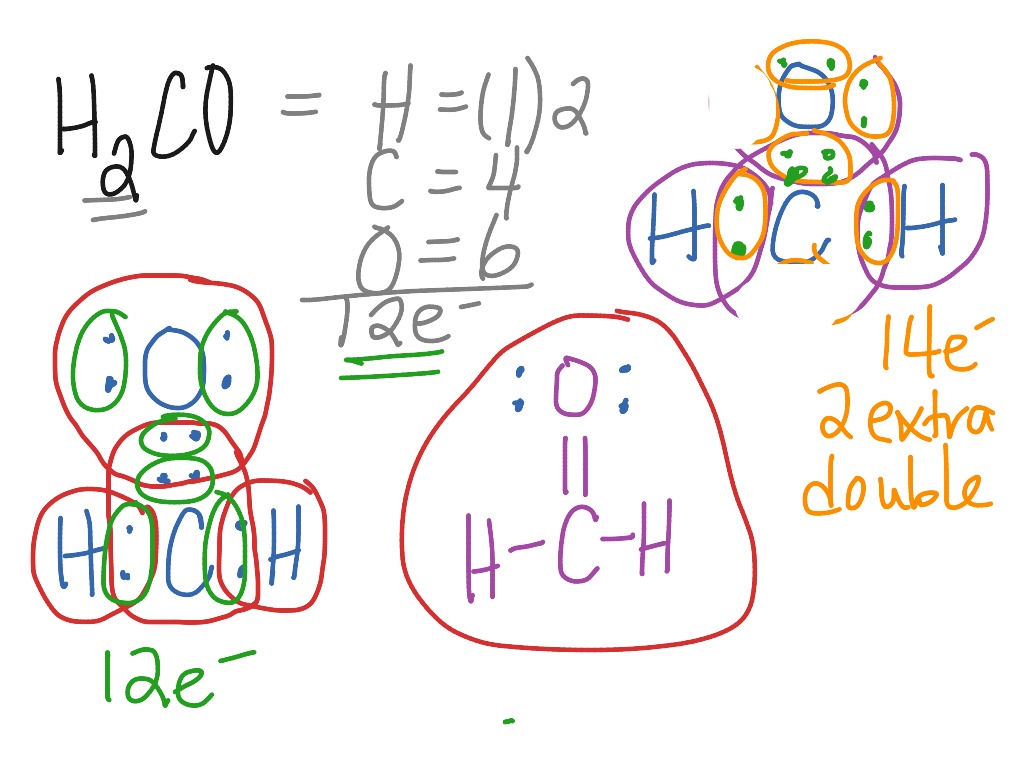Have you ever looked at a molecule and wondered how its atoms connect, or what shape it takes in space? Well, for many, the world of molecular structures can feel a bit like cracking a secret code. But it's actually pretty cool, and understanding these blueprints helps us see how chemicals behave. Today, we're going to take a really close look at the H2CO Lewis structure, which is the foundational way we map out formaldehyde, a chemical you might hear about in everyday life.
Formaldehyde, or H2CO, is a simple organic compound, yet it holds some very important lessons about chemical bonding and molecular shapes. Learning how to draw its Lewis structure is, in a way, like learning the alphabet of molecular chemistry. It helps you figure out not just how the atoms are arranged, but also how they share electrons, which then tells us so much more about the molecule's properties.
So, if you're curious about how atoms come together to form compounds, or if you're just trying to get a better handle on Lewis structures, this guide is definitely for you. We'll walk through everything, from counting electrons to figuring out its exact shape, and you'll see how it all fits together, very naturally.
Table of Contents
- What is Formaldehyde (H2CO)?
- Drawing the H2CO Lewis Structure: A Step-by-Step Guide
- Unpacking H2CO's Geometry: VSEPR Theory in Action
- Hybridization of Carbon in H2CO
- Understanding Bonds: Sigma and Pi in H2CO
- Intermolecular Forces in Formaldehyde
- Frequently Asked Questions About H2CO Lewis Structure
What is Formaldehyde (H2CO)?
Formaldehyde, known by its chemical formula H2CO, is a really simple organic compound. It's the simplest aldehyde, actually, and it's quite important in various industries. You might have heard of it because it's used in making building materials, like particleboard and plywood, and also in many household products. It's also used as a preservative, which is kind of interesting, for biological specimens, for example. Its structure, which we're about to explore, helps explain why it behaves the way it does, very much.
Understanding the H2CO Lewis structure gives us a visual representation of how the atoms within this molecule are connected. It shows us which atoms are bonded to each other and how the electrons are distributed around them. This visual map is a foundational step for predicting its shape, its reactivity, and even how it interacts with other molecules, you know.
Drawing the H2CO Lewis Structure: A Step-by-Step Guide
Drawing a Lewis structure is a bit like following a recipe. There are clear steps to take, and if you follow them, you'll get the correct picture of the molecule's electron arrangement. For H2CO, it's a pretty straightforward process, so let's get started, shall we?
- 636 Angel Number
- Different Types Of Hats
- Fondos De Pantalla Aesthetic
- Taylor Swift Gif
- Watermelon Clipart
Counting Valence Electrons
The very first step in drawing any Lewis structure, including H2CO, is to figure out the total number of valence electrons available. These are the electrons in the outermost shell of an atom, and they're the ones involved in forming chemical bonds. For H2CO, we have carbon (C), oxygen (O), and hydrogen (H) atoms. Carbon is in Group 14, so it has 4 valence electrons. Oxygen is in Group 16, giving it 6 valence electrons. Each hydrogen atom, being in Group 1, contributes 1 valence electron. Since there are two hydrogen atoms, they contribute 2 valence electrons in total. So, we add them all up: 4 (from C) + 6 (from O) + 2 * 1 (from 2 H) = 12 total valence electrons. This number is really important, you know, because it's the total number of electrons we have to work with.
Identifying the Central Atom
Next, we need to pick which atom will sit in the middle of our structure. Typically, the central atom is the least electronegative atom (except for hydrogen, which is always a terminal atom because it can only form one bond). In H2CO, carbon is less electronegative than oxygen. Hydrogen can only form one bond, so it can never be central. This means carbon (C) will be our central atom, with the two hydrogen atoms and one oxygen atom attached to it. This arrangement is pretty typical for organic molecules where carbon is often the backbone, actually.
Connecting Atoms with Single Bonds
Once you've identified the central atom, the next move is to connect all the other atoms to it using single bonds. Each single bond represents two shared electrons. For H2CO, we'll draw a single bond between carbon and each of the two hydrogen atoms, and another single bond between carbon and the oxygen atom. So, that's three single bonds in total. Since each bond uses 2 electrons, we've used 3 * 2 = 6 electrons so far. We started with 12 total valence electrons, so now we have 12 - 6 = 6 electrons left to distribute. This step helps establish the basic skeleton of the molecule, you see.
Completing Octets for Outer Atoms
After placing the initial single bonds, the next step is to make sure the outer atoms (also called terminal atoms) have a complete octet of electrons. An octet means 8 electrons, which makes an atom stable, generally speaking. Hydrogen is an exception; it only needs 2 electrons to be stable (a duet). In H2CO, both hydrogen atoms already have 2 electrons from their single bond with carbon, so they are stable. Oxygen, however, needs an octet. It currently has 2 electrons from its single bond with carbon. To reach 8, it needs 6 more electrons. We'll add these 6 electrons as three lone pairs around the oxygen atom. So, we've used all 6 of our remaining electrons on the oxygen atom. Now, all outer atoms are satisfied, which is good.
Placing Remaining Electrons on the Central Atom
After satisfying the octets (or duets for hydrogen) of the outer atoms, any leftover valence electrons are placed on the central atom. In our H2CO example, we started with 12 electrons, used 6 for single bonds, and then used the remaining 6 on the oxygen atom. This means we have 0 electrons left over. So, there are no lone pairs to place on the central carbon atom. This is an important detail, as the presence or absence of lone pairs on the central atom significantly impacts the molecular geometry, as we'll see very soon.
Forming Multiple Bonds if Needed
The final check is to ensure that the central atom also has a complete octet. Let's look at our carbon atom in H2CO. It's currently bonded to two hydrogens and one oxygen with single bonds. This means it has 2 (from C-H) + 2 (from C-H) + 2 (from C-O) = 6 electrons around it. Carbon needs 8 electrons to complete its octet. Since carbon is short 2 electrons, and oxygen has lone pairs available, we can move one of the lone pairs from the oxygen atom to form an additional bond with carbon. This creates a double bond between carbon and oxygen. Now, carbon has 2 (from C-H) + 2 (from C-H) + 4 (from C=O) = 8 electrons, completing its octet. Oxygen now has 4 electrons from the double bond and 4 electrons from its two remaining lone pairs, also completing its octet. This is the correct and most stable Lewis structure for H2CO, very much so. It shows a carbon atom double-bonded to an oxygen atom and single-bonded to two hydrogen atoms, with two lone pairs on the oxygen.
Unpacking H2CO's Geometry: VSEPR Theory in Action
Once you have the Lewis structure, you can figure out the molecule's three-dimensional shape using something called VSEPR theory. VSEPR stands for Valence Shell Electron Pair Repulsion. It's a fancy way of saying that electron groups around a central atom will arrange themselves as far apart as possible to minimize repulsion. This repulsion is what dictates the molecule's geometry, you know.
Electron Domain Geometry
First, we look at the electron domain geometry around the central carbon atom. An electron domain is simply a region where electrons are found. This can be a single bond, a double bond, a triple bond, or a lone pair of electrons. In our H2CO Lewis structure, the central carbon atom has three electron domains: two single bonds (to hydrogen) and one double bond (to oxygen). Since there are no lone pairs on the central carbon, all three domains are bonding domains. With three electron domains around the central atom, VSEPR theory predicts a trigonal planar electron domain geometry. This means these three electron groups will spread out in a flat plane, trying to get as much space from each other as possible, you see.
Molecular Geometry
The molecular geometry describes the arrangement of only the atoms in space, ignoring any lone pairs on the central atom. Since the central carbon in H2CO has no lone pairs, its electron domain geometry and its molecular geometry are the same. Therefore, the molecular geometry of H2CO is also trigonal planar. This means the carbon atom, the two hydrogen atoms, and the oxygen atom all lie in the same flat plane. It's a pretty neat, flat molecule, actually.
Bond Angles
In a perfect trigonal planar arrangement, the ideal bond angles are 120 degrees. For H2CO, because the carbon-oxygen double bond is a bit "fatter" than the carbon-hydrogen single bonds (it has more electron density), it tends to push the C-H bonds slightly closer together. So, while the O=C-H bond angles are approximately 120 degrees, the H-C-H bond angle might be slightly less than 120 degrees, perhaps around 116-118 degrees, and the O=C-H angles would be slightly larger to compensate. But for general purposes, it's often approximated as 120 degrees. This slight distortion is a subtle detail that shows how real molecules can deviate a little from ideal shapes, very much.
Hybridization of Carbon in H2CO
Hybridization is a concept that helps explain how atoms form bonds and achieve their specific geometries. It involves the mixing of atomic orbitals to create new, hybrid orbitals that are better suited for bonding. For the central carbon atom in H2CO, we have three electron domains. When an atom has three electron domains, it typically undergoes sp2 hybridization. This means one 's' orbital and two 'p' orbitals from the carbon atom mix to form three new sp2 hybrid orbitals. These three sp2 hybrid orbitals point towards the corners of an equilateral triangle, explaining the trigonal planar geometry. The remaining unhybridized 'p' orbital on the carbon atom is then available to form the pi bond with oxygen. So, the carbon in H2CO is sp2 hybridized, which makes a lot of sense given its shape, you know.
Understanding Bonds: Sigma and Pi in H2CO
Chemical bonds aren't all the same; they have different characteristics. In H2CO, we have both single and double bonds, which means we'll find both sigma (σ) and pi (π) bonds. Understanding these types of bonds helps explain the molecule's stability and reactivity, very simply.
Counting Sigma Bonds
A sigma bond is the first bond formed between any two atoms and results from the direct, head-on overlap of atomic orbitals. Every single bond is a sigma bond. In a double bond, one of the bonds is a sigma bond, and the second bond is a pi bond. In H2CO, there are two C-H single bonds, and one C=O double bond. This means we have:
- One sigma bond for each C-H single bond (2 total)
- One sigma bond within the C=O double bond
Orbital Overlap in H2CO
Let's look at how these sigma bonds are formed through orbital overlap.
- C-H Sigma Bonds: Each C-H sigma bond is formed by the overlap of an sp2 hybrid orbital from the carbon atom and a 1s orbital from each hydrogen atom. The sp2 orbitals of carbon are quite good at forming these direct overlaps, providing a strong connection, you see.
- C=O Bonds: The carbon-oxygen double bond is a bit more involved. One part of this double bond is a sigma bond, formed by the overlap of an sp2 hybrid orbital from carbon and an sp2 hybrid orbital from oxygen. Oxygen, too, undergoes sp2 hybridization because it also has three electron domains (one bonding to carbon, and two lone pairs). The second part of the C=O double bond is a pi bond. This pi bond is formed by the side-by-side overlap of the unhybridized 2p orbital from carbon and an unhybridized 2p orbital from oxygen. These pi bonds are usually weaker than sigma bonds but add to the overall strength and rigidity of the double bond. This combination of sigma and pi bonds is what gives the C=O group its characteristic properties, very much so.
Intermolecular Forces in Formaldehyde
Intermolecular forces are the attractions between different molecules, not within the molecule itself. These forces determine many physical properties, like boiling point and solubility. For H2CO, we need to consider its polarity. Because of the highly electronegative oxygen atom and the double bond, the C=O bond is quite polar. This creates a net dipole moment for the entire H2CO molecule, meaning it has a slightly positive end and a slightly negative end. As a result, H2CO molecules experience dipole-dipole forces, where the positive end of one molecule is attracted to the negative end of another. Additionally, all molecules, including H2CO, experience London dispersion forces (also called induced dipole-induced dipole forces), which are temporary attractions arising from instantaneous shifts in electron distribution. So, H2CO experiences both London dispersion forces and dipole-dipole forces. It does not, however, typically form hydrogen bonds with other H2CO molecules. This is because hydrogen bonding requires a hydrogen atom directly bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine, and while H2CO has oxygen, its hydrogens are bonded to carbon, not oxygen. This is a common point of confusion, but it's important to remember, you know.
Understanding the H2CO Lewis structure and its related properties really opens up a lot about how molecules work. From the initial electron count to the final shape and how molecules interact, each step builds on the last, giving us a complete picture. It's quite a fundamental example in chemistry, and mastering it helps with so many other molecular structures. You can learn more about molecular structures on our site, and perhaps explore other related topics like chemical bonding principles for a deeper dive.
Frequently Asked Questions About H2CO Lewis Structure
Here are some common questions people ask about formaldehyde's structure, you know.
How do you draw the Lewis structure of H2CO?
To draw the H2CO Lewis structure, you first count all valence electrons (12 for H2CO). Then, you identify carbon as the central atom. Connect the two hydrogen atoms and one oxygen atom to the carbon with single bonds. Distribute remaining electrons to complete octets on outer atoms (oxygen gets 6 lone pair electrons). Finally, if the central carbon doesn't have an octet, move a lone pair from oxygen to form a double bond with carbon, ensuring both carbon and oxygen have complete octets. This systematic approach really helps, actually.
What is the molecular geometry of H2CO?
The molecular geometry of H2CO is trigonal planar. This is because the central carbon atom has three electron domains (two single bonds to hydrogen and one double bond to oxygen) and no lone pairs. According to VSEPR theory, these three electron domains repel each other and arrange themselves as far apart as possible in a flat, triangular shape, resulting in bond angles of approximately 120 degrees. It's a very flat molecule, you see.
What is the hybridization of carbon in H2CO?
The carbon atom in H2CO is sp2 hybridized. This occurs because the carbon atom has three electron domains around it. One 's' atomic orbital mixes with two 'p' atomic orbitals to form three new sp2 hybrid orbitals. These sp2 hybrid orbitals are used to form the three sigma bonds (two with hydrogen and one with oxygen). The remaining unhybridized 'p' orbital on carbon then forms the pi bond with oxygen. This hybridization perfectly matches the trigonal planar geometry, very much so.